Dr. Andrew Weems spotlights the role of “blebs” in cancer cell signaling
JCC Fellow Dr. Andrew Weems recently published his latest findings investigating the role of cellular blebs in oncogenic cellular signaling. Cancer cells are often covered in blebs, small round bumps thought to aid metastasis through enabling cell motility. Weems and colleagues hypothesized that blebs might play additional roles in enabling cancer cell survival, as they observed that proteins and lipids involved in survival signaling were highly concentrated within blebs. Indeed, they found that inhibiting bleb formation, through either pharmacological or mechanical means, killed melanoma cells. But how were blebs promoting the survival signals that were keeping these cancer cells alive? Here, Weems drew on his PhD experience for key insight into the downstream factors.
Pursuing his passions, from art to science
Dr. Weems grew up in the small town of Katy, Texas. In high school his interests surrounded acting, dancing, and ballet. This passion for the arts helped him get through high school and led him to Austin post graduation. After a few years in the Austin arts scene, he did not see much of a future in continuing in that direction. In search of his next step, Weems started taking classes at Austin Community College towards a certificate in radiologic technology. There, he discovered a love and a knack for cell biology. After finishing his undergraduate studies at Texas State University under the mentorship of Dr. Joseph Koke, Weems joined Dr. Michael McMurray’s lab at the University of Colorado Anschutz Medical Campus.

Weems was the first graduate student in Dr. McMurray’s lab. He credits McMurray with helping him to appreciate the puzzle of science: forming a hypothesis, designing and analyzing experiments to rigorously refine that hypothesis, rinse and repeat. In McMurray’s lab, Weems worked on the puzzle of septin assembly. Septins are rod-shaped assemblies constructed using five potential protein building blocks. However, it wasn’t clear if there was any specificity for which of these blocks were used during septin assembly. Weems developed a clever and novel in vivo assay, and used it to determine that distinct GTP-hydrolysis rates between the possible septin building blocks dictate the identity of their local binding partners. Thus, septin oligomers assemble via ordered pathways which are regulated by the metabolic state of the cell (i.e. GTP/GDP ratio).
As Dr. Weems finished his PhD, he next looked for a lab to conduct his postdoctoral research. Weems enjoyed the cellular biology work of his PhD, and wanted to find a lab where he could do quantitative microscopy. Ideally, he wanted to work in a lab that combined incredible microscopy technology with biologists asking interesting mechanistic questions. Weems found that unique combination in Dr. Gaudenz Danuser’s lab at UT Southwestern Medical Center. Weems was hooked after interviewing in the Danuser lab and hearing about their weird burgeoning project connecting bleb formation and cell signaling.
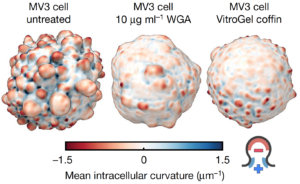
Bleb formation constructs septin-signaling hubs that promote melanoma cell survival
Based on his past research experience, Weems surmised that blebs might recruit septins to the cell surface since they generate plasma membrane curvature that septins bind to. Also, because septins form signaling hubs capable of regulating the survival signals they had observed near blebs in these cells, septins could be the missing link connecting blebbing and the survival signaling that was keeping these cells alive. Consistent with this hypothesis, Weems found septin enrichment in blebs. Furthermore, inhibiting septin activity disrupted downstream survival signaling and killed melanoma cells. Therefore, Weems and colleagues think that bleb formation provides additional context for understanding how oncogenic genetic mutations lead to carcinogenesis. In the melanoma cells being studied, there is a mutation in the signaling protein NRAS. Mutations in NRAS and related proteins are very common in melanoma and many other types of cancer. Thus, Weems’ findings suggest that NRAS mutations are not sufficient on their own to promote oncogenic NRAS signaling, but additionally require a permissive cell morphological state (bleb formation).
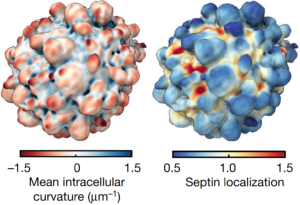
These findings suggest that cell morphology may not just be a readout of cancer cells and oncogenic signaling. Rather, bleb morphology directly contributes to the activated survival signaling observed in cancer cells. Next, Weems investigated whether bleb signaling is a peculiarity specific to melanoma, or rather, if it is a conserved developmental process that could be readily co-opted by many types of cancer. Indeed, he found that normal cells lacking oncogenic mutation also use blebs to build septin-signaling hubs that promote their survival. Thus, Weems thinks that septin-mediated bleb signaling is likely to be a common survival strategy for many types of cancers.
Dr. Weems’ next act
Moving forward, Dr. Weems is looking to start his own lab investigating how widespread bleb signaling is in different cancers, the ways in which bleb signaling might contribute to cancer progression, and what roles bleb signaling plays in normal cells. Additionally, Weems wants to investigate if septin inhibition is a viable drug target in any of these diseases. RAS mutations, including the NRAS variety found in Weems’ melanoma cells, are notoriously difficult to inhibit. Thus, Weems suggests that septins may represent a more tractable drug target for many types of cancer.
Dr. Weems is grateful to JCC for their support of his postdoctoral research. He notes that the JCC fellowship was a reassuring confidence boost in the early days of his postdoc. This boost helped him persevere through the normal self-doubt and pressure involved in high-risk/high-reward research. Additionally, Weems absolutely loved attending the JCC annual meeting. He advises new fellows that the annual meeting is “a feast, don’t eat beforehand!” (Weems is currently brainstorming how he can attend the annual meeting every year to get his fill.) He notes that the scientific input and feedback he received from his JCC cohort have been invaluable. Thus, Weems encourages JCC Fellows to maximize the immense resource of their colleagues’ experience and thoughtfulness.