Small molecules can interfere with dengue virus entry
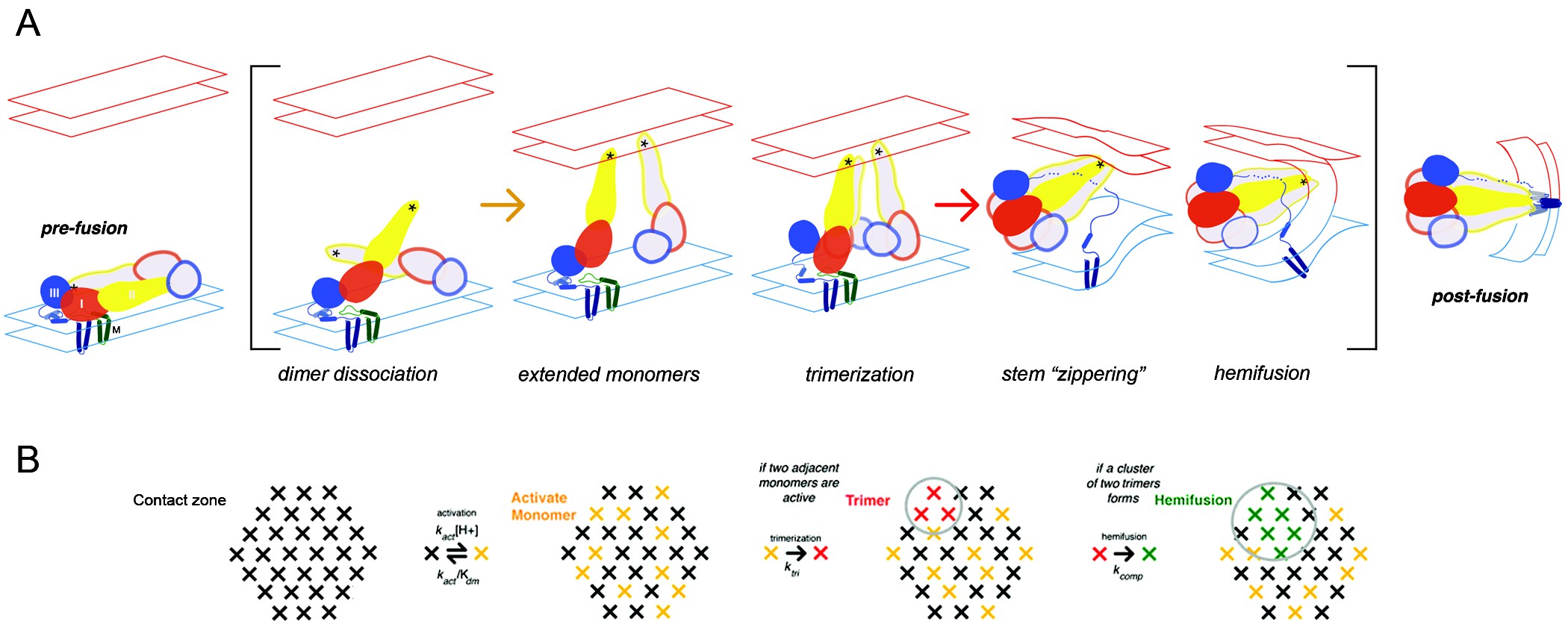
Caption: Flavivirus membrane fusion. A) Steps in the fusion transition. Starting point and end point represent known structures; steps in brackets inferred from experimental evidence as summarized in (Chao et al., 2014). B) Scheme for simulation of fusion reaction. Array of crosses represents a contact zone with 30 monomers. Image Credit Chao et al (CC BY 4.0) https://doi.org/10.7554/eLife.36461.002
When the virus that causes Dengue fever infects a cell, its protective envelope first binds with the outside of the cell membrane. The cell engulfs the virus, surrounding it in an endosome compartment. Then, the viral envelope fuses with the endosome membrane, allowing viral RNA to infect the cell. All flaviviruses, including West Nile, Zika, and Yellow Fever, follow a similar path into the cell.
“The fusion step is critical for the virus to deliver its infectious cargo to the cell,” says Luke Chao, assistant professor of genetics at Harvard Medical School, and requires “molecular acrobatics by the viral proteins.”
Earlier work had shown that the West Nile virus’s envelope protein (E) is tiled in pairs on the viral surface. The acidic endosome environment causes E protein pairs to separate and move toward the endosome membrane, where they forms groups of three. In a previous West Nile virus study by Chao and his colleagues, researchers tracked individual viral-particles to determine which step controlled the overall speed of fusion. They found evidence that the later steps in forming groups of three are rate-limiting in fusing the virus with the endosome membrane, to infect the cell.
But was the process the same in the Dengue virus? And could it be interrupted? In a recent follow-up study published in eLife, Chao and his colleagues demonstrate that yes, the process was similar in Dengue, and described how a small molecule inhibitor interferes with membrane fusion.
“We were interested in knowing how many copies of the small molecules are required to prevent entry into the cell,” says Chao, who completed this research and his JCC Fellowship with Stephen Harrison, professor of biological chemistry at Harvard Medical School. “And we found that you could stop the virus even if you don’t block all the potential binding sites on the proteins.”
Imagine a sporting event, Chao explains. When a player scores a goal, people in a crowd will all stand up and cheer. But if one person refrains from such an enthusiastic display, their neighbors might stop clapping as well. “You don’t have to stop every person from clapping to interfere with the process,” he says.
Blocking only 25% of the envelope proteins on a virus could completely block fusion.
The study was a collaboration among scientists in diverse fields. In addition to the Harrison lab, the labs of Nathanael Gray at the Dana Farber Cancer Institute and Priscilla Yang at the Harvard Medical School also contributed to the research.
“These particular small molecules are useful tools — not necessarily compounds destined for clinical trials. But they are handy tools because they allow us to answer mechanistic questions at a molecular level that would be difficult to answer in animal models” says Chao, explaining that these small molecule tools were developed in Yang’s lab. “Understanding this molecular interaction between the viral envelope, the cell membrane, and small molecules is critical to developing drugs in the future.”
This research is ongoing in the Harrison lab, with scientists continuing to investigate flaviviruses’s entry into cells. Now that Chao leads his own lab, he has shifted his focus to membrane fusion between mitochondria, an event that is misregulated in cancer and neurodegenerative diseases.