“A totally new angle in tissue engineering”
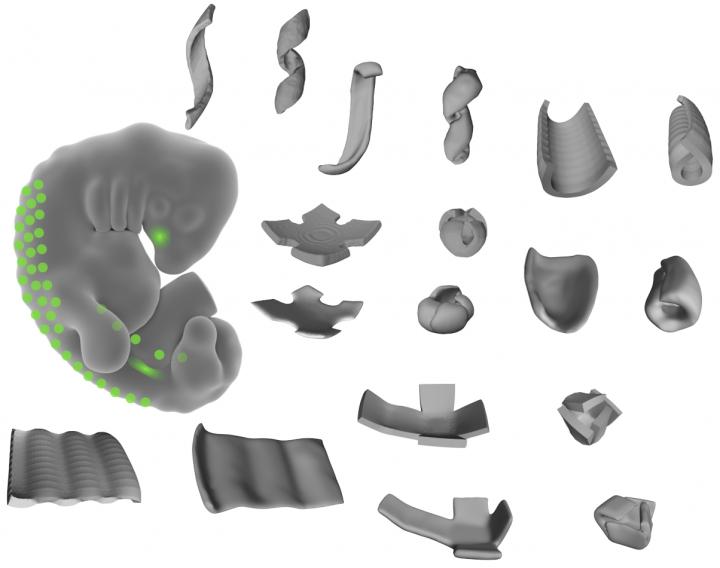
Caption: This image shows the shapes made of living tissue made by the researchers. By patterning mechanically active mouse or human cells to thin layers of extracellular fibers, the researchers could create bowls, coils, and ripple shapes. Credit: Alex Hughes
By manipulating combinations of living cells within extracellular matrices of proteins and other components, former JCC Fellow Alex Hughes (2015-2018) and his colleagues created different shapes of living tissue. The method is quite different from other types of tissue engineering, which use a top down approach to design, says Hughes, who completed his postdoctoral fellowship at UCSF with Zev Gartner.
“This new philosophy suggests that building replacement tissue needs to follow developmental principals, from blueprints that we can control using engineering tools,” he says, explaining that previous work used a fixed scaffold and coaxed cells to grow in that predetermined shape. “We propose that tissue engineers harness developmental principals rather than try to override natural principals that cells use.”
This study, published in Developmental Cell, focused on how cells communicate mechanically during embryonic developmental processes. When Hughes placed clusters of mouse embryo cells in an extracellular matrix, he found that cells pulled on the matrix, altering its shape. Changing the pattern of the cell clusters changed the types of shapes produced. In this way, Hughes created bowls, coils, and ripples of living tissue.
“We were shocked, initially,” says Hughes. He didn’t expect individual cells to generate force to change the structure’s shape. “But we saw radical folding events from just a few cells.” Imagine, he explains, a group of people with their arms linked. Flexing your arm could make you fold in on your neighbor and change the shape of the group slightly. But if everyone pulls in at the same time, the entire group would contract quickly.
“We’re beginning to see that it’s possible to break down natural developmental processes into engineering principles that we can then repurpose to build and understand tissues,” Hughes says. “It’s a totally new angle in tissue engineering.”
Hughes recently left his postdoc to begin an assistant professorship at Penn, where he studies “how cells conspire to create complex structures, so that we can build models of human disease, reconstituted tissues for regenerative medicine, and new types of biological machines.”
He credits his JCC fellowship for allowing him freedom and autonomy to pursue interdisciplinary research — and connecting him with top scientists in fields relevant to his work. “Science is about people, and JCC is critical for connecting people,” he says.